浙江农业学报 ›› 2025, Vol. 37 ›› Issue (12): 2468-2478.DOI: 10.3969/j.issn.1004-1524.20240730
十足目虹彩病毒1和虾肝肠胞虫双重荧光定量PCR检测方法的建立
- 1.绍兴文理学院 生命与环境科学学院,浙江 绍兴 312000
2.浙江华珍科技有限公司,浙江 诸暨 311804
-
收稿日期:2024-08-13出版日期:2025-12-25发布日期:2026-01-09 -
作者简介:谈荣想(1999—),男,江西瑞昌人,硕士研究生,研究方向为水生动物病害与免疫学。E-mail:2395774885@qq.com -
通讯作者:*许婷,E-mail:xuting@usx.edu.cn
Establishment of a double quantitative PCR method for the detection of Decapod iridescent virus 1 and Enterocytozoon hepatopenaei
TAN Rongxiang1( ), SI Guangjie2, SUN Haitao2, XU Ting1,*(
), SI Guangjie2, SUN Haitao2, XU Ting1,*( )
)
- 1. School of Life and Environmental Sciences, Shaoxing University, Shaoxing 312000, Zhejiang, China
2. Zhejiang Huazhen Sci & Tech Co., Ltd., Zhuji 311804, Zhejiang, China
-
Received:2024-08-13Online:2025-12-25Published:2026-01-09
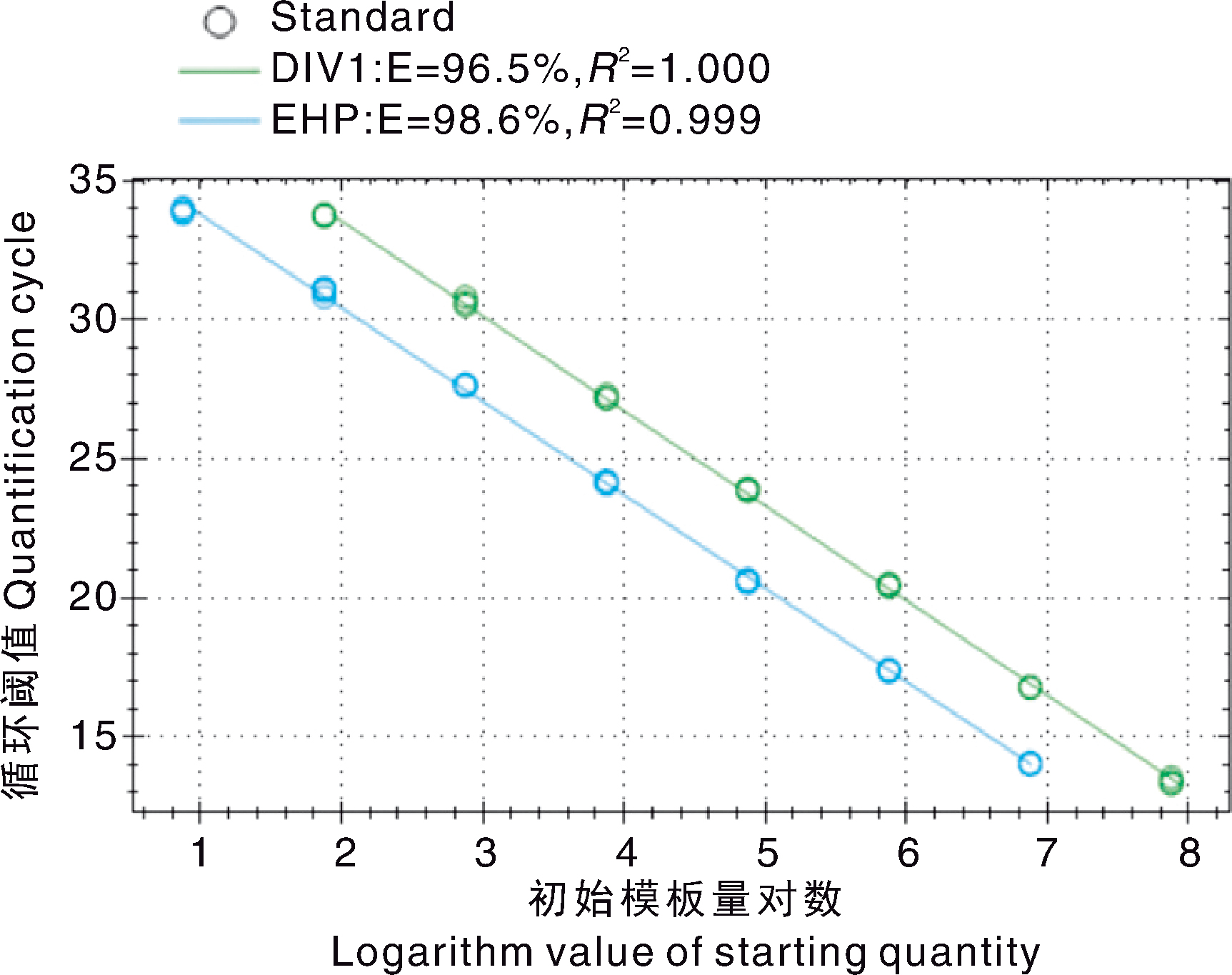
摘要: 为建立一种可同时检测十足目虹彩病毒1(Decapod iridescent virus 1, DIV1)与虾肝肠胞虫(Enterocytozoon hepatopenaei, EHP)的方法,本研究在已有DIV1实时荧光定量PCR(quantitative real-time PCR, qPCR)检测方法的基础上,开发了一种基于SYBR Green I的双重qPCR方法,分别靶向DIV1的主要衣壳蛋白(major capsid protein, MCP)基因和EHP的孢壁蛋白(spore wall protein, SWP)基因。结果显示,该方法中DIV1与EHP的熔解温度(Tm)分别为(82.0±0.5)℃和(77.0±0.5)℃,能够特异性检出DIV1和EHP,而对其他常见虾病原和健康虾样本的检测结果均为阴性;在35个循环内,DIV1与EHP的最低检测限分别为75 copies·μL-1和15 copies·μL-1;重复性试验显示,组内和组间变异系数均低于2%。综上,本研究建立的双重qPCR检测方法具有高度的特异性、灵敏度与重复性,适用于虾中DIV1和EHP感染的快速监测。
中图分类号:
引用本文
谈荣想, 斯广杰, 孙海涛, 许婷. 十足目虹彩病毒1和虾肝肠胞虫双重荧光定量PCR检测方法的建立[J]. 浙江农业学报, 2025, 37(12): 2468-2478.
TAN Rongxiang, SI Guangjie, SUN Haitao, XU Ting. Establishment of a double quantitative PCR method for the detection of Decapod iridescent virus 1 and Enterocytozoon hepatopenaei[J]. Acta Agriculturae Zhejiangensis, 2025, 37(12): 2468-2478.
| 引物名称 Primer name | 引物序列(5'→3') Primer sequence(5'→3') | 扩增片段长度 Product length/bp | 目的 Purpose |
|---|---|---|---|
| DIV1-QF | GCCATTCCCGAACTCACC | 154 | 双重qPCR方法检测DIV1 |
| DIV1-QR | CTTCACCCTTTGCCGCTT | Detection of DIV1 by double qPCR method | |
| SWP-stdF | TTAAGTAATTACGAGTTTGGC | 318 | 构建pMD-SWP标准质粒 |
| SWP-stdR | GTTATTTACAGTTTTGCGTTG | Construction of pMD-SWP standard plasmid | |
| EHP-QF | TGGCGGCACAATTCTCAAACAT | 102 | 双重qPCR方法检测EHP |
| EHP-QR | GCTGTGTCTGTGTAAATATCGTC | Detection of EHP by double qPCR method |
表1 本研究的引物序列信息
Table 1 Sequences of primers used in this study
| 引物名称 Primer name | 引物序列(5'→3') Primer sequence(5'→3') | 扩增片段长度 Product length/bp | 目的 Purpose |
|---|---|---|---|
| DIV1-QF | GCCATTCCCGAACTCACC | 154 | 双重qPCR方法检测DIV1 |
| DIV1-QR | CTTCACCCTTTGCCGCTT | Detection of DIV1 by double qPCR method | |
| SWP-stdF | TTAAGTAATTACGAGTTTGGC | 318 | 构建pMD-SWP标准质粒 |
| SWP-stdR | GTTATTTACAGTTTTGCGTTG | Construction of pMD-SWP standard plasmid | |
| EHP-QF | TGGCGGCACAATTCTCAAACAT | 102 | 双重qPCR方法检测EHP |
| EHP-QR | GCTGTGTCTGTGTAAATATCGTC | Detection of EHP by double qPCR method |
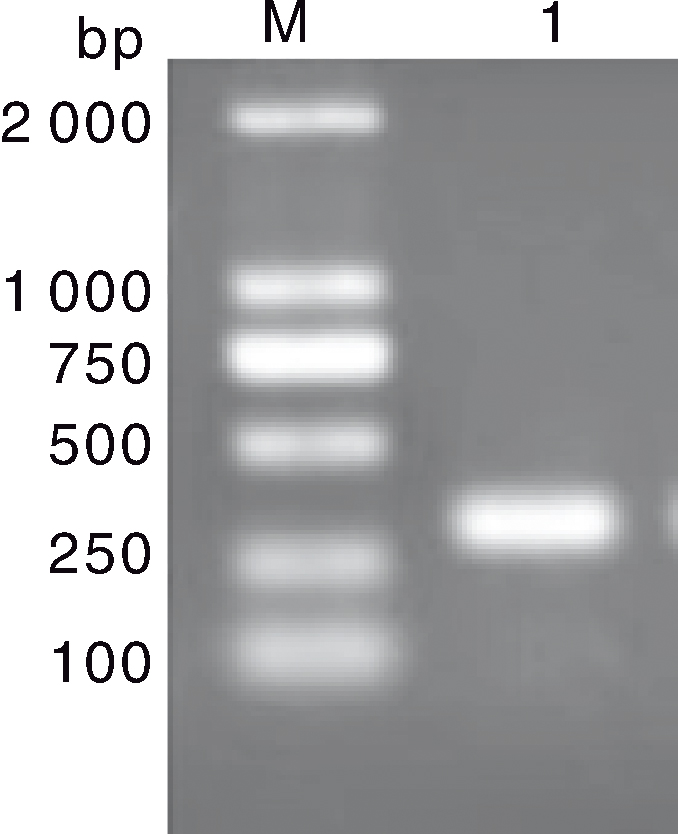
图1 虾肝肠胞虫SWP基因的PCR扩增结果 M, DL2000 DNA marker;1, SWP基因扩增产物。
Fig.1 PCR amplification results of SWP gene from Enterocytozoon hepatopenaei M, DL2000 DNA marker; 1, PCR amplification products of SWP gene.
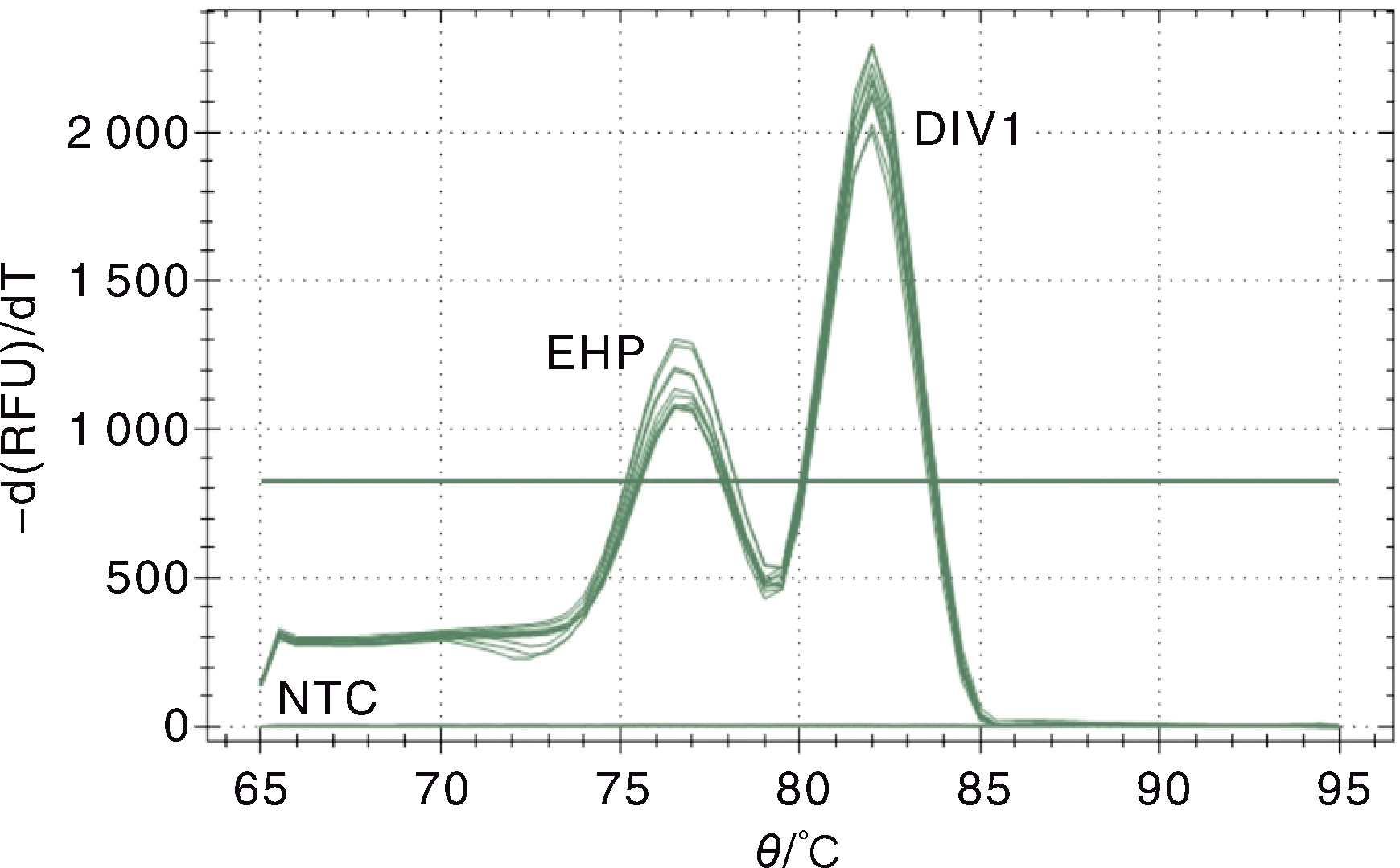
图2 十足目虹彩病毒1与虾肝肠胞虫的双重SYBR Green I qPCR熔解曲线 EHP,EHP标准质粒pMD-SWP的熔解峰;DIV1,DIV1标准质粒pMD-MCP的熔解峰;NTC,阴性对照。
Fig.2 Melting curve of the double SYBR Green I qPCR for Decapod iridescent virus 1 (DIV1) and Enterocytozoon hepatopenaei(EHP) EHP, The melting curve of EHP standard plasmid pMD-SWP; DIV1,The melting curve of DIV1 standard plasmid pMD-MCP; NTC,Negative control.
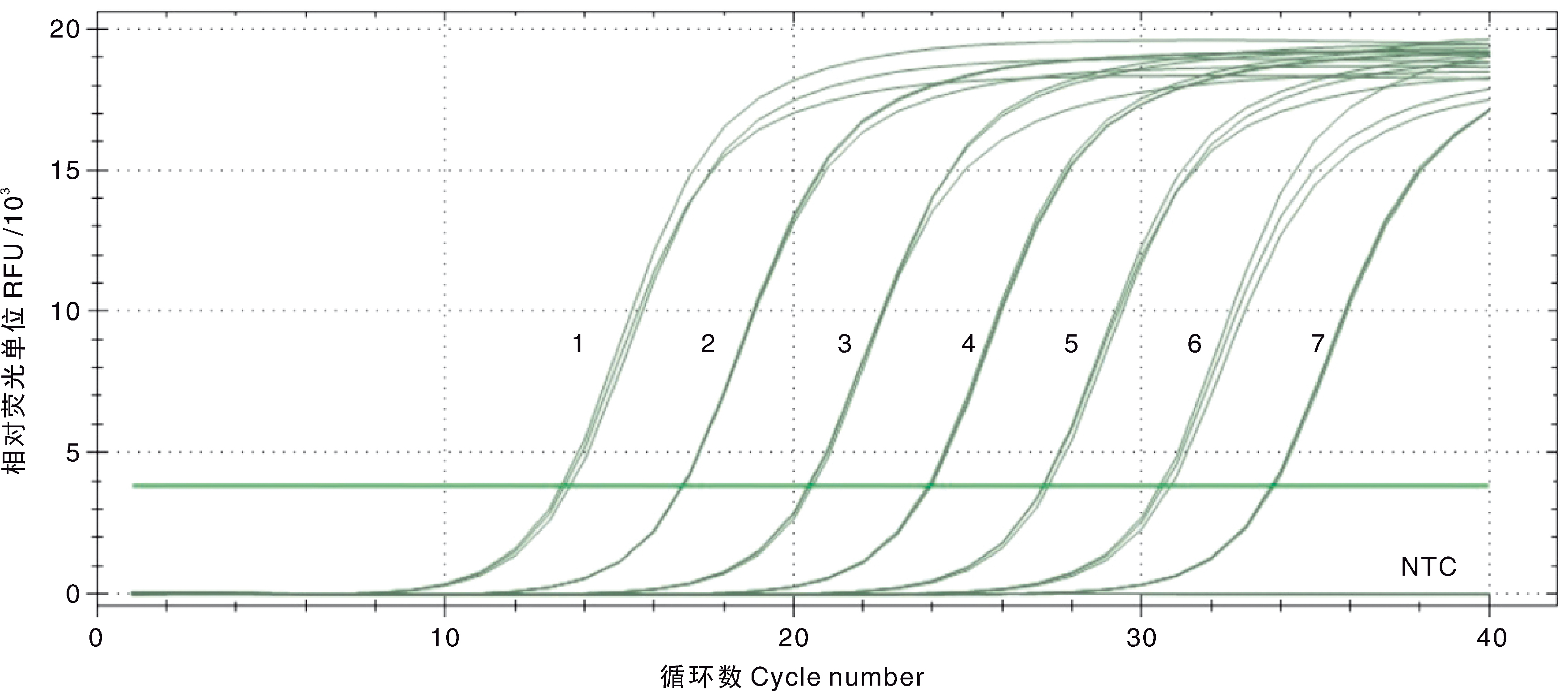
图3 DIV1双重SYBR Green I qPCR扩增曲线和灵敏性试验结果 1~7,7.5×107~7.5×101 copies·μL-1的pMD-MCP系列标准品;NTC,阴性对照。
Fig.3 Amplification curve and sensitivity of the double SYBR Green I qPCR for DIV1 1-7, 7.5×107-7.5×101 copies·μL-1 of the standard plasmids pMD-MCP; NTC, Negative control.
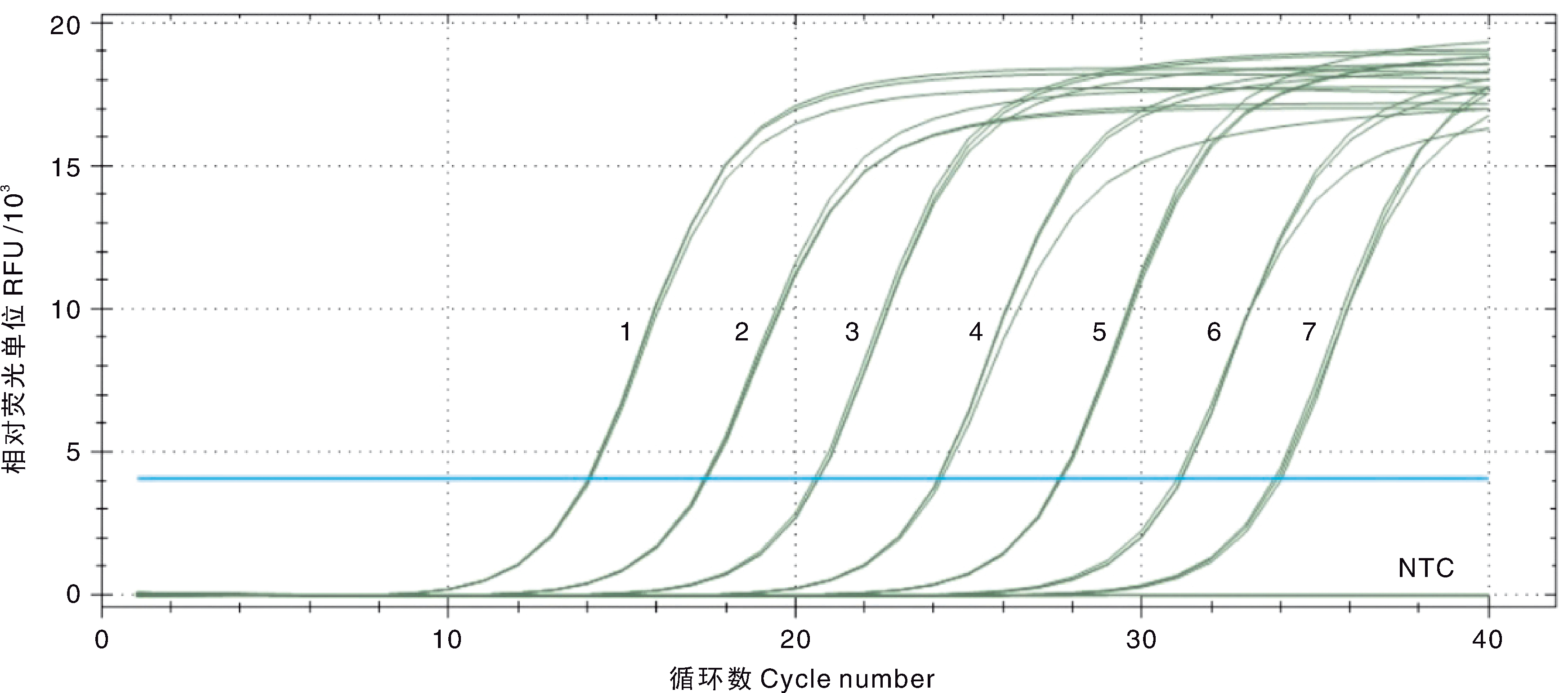
图4 EHP双重SYBR Green I qPCR扩增曲线和灵敏性试验结果 1~7,1.5×107~1.5×101 copies·μL-1的pMD-SWP系列标准品;NTC,阴性对照。
Fig.4 Amplification curve and sensitivity of the double SYBR Green I qPCR for EHP 1-7, 1.5×107-1.5×101 copies·μL-1 of the standard plasmids pMD-SWP; NTC, Negative control.
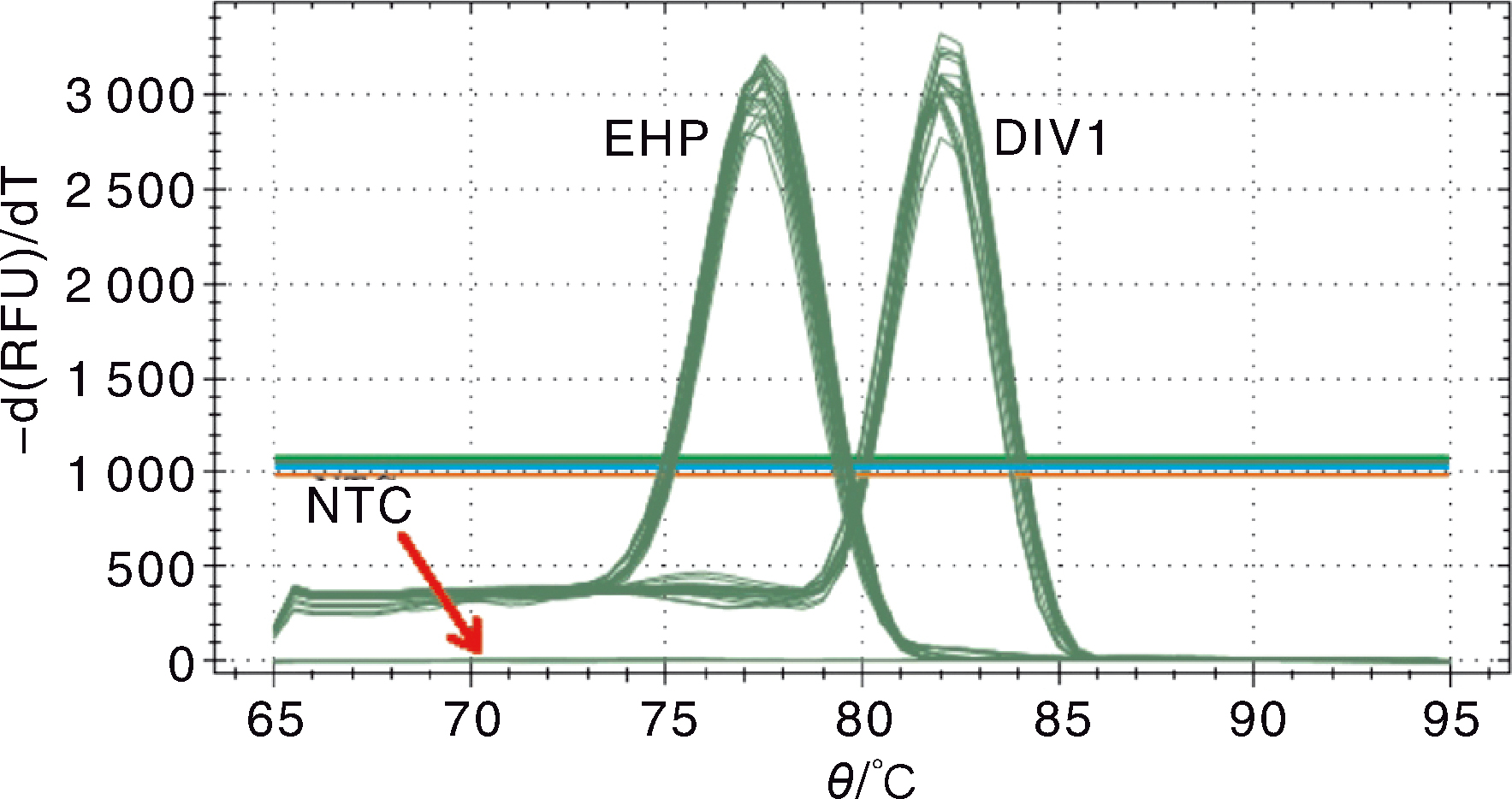
图6 DIV1和EHP双重SYBR Green I qPCR检测的熔解曲线 EHP,梯度稀释的pMD-SWP系列标准品;DIV1,梯度稀释的pMD-MCP系列标准品;NTC,阴性对照。
Fig.6 Melting curve for the double SYBR Green I qPCR for DIV1 and EHP EHP, Serial dilution of standard plasmid pMD-SWP; DIV1, Serial dilution of standard plasmid pMD-MCP; NTC, Negative control.
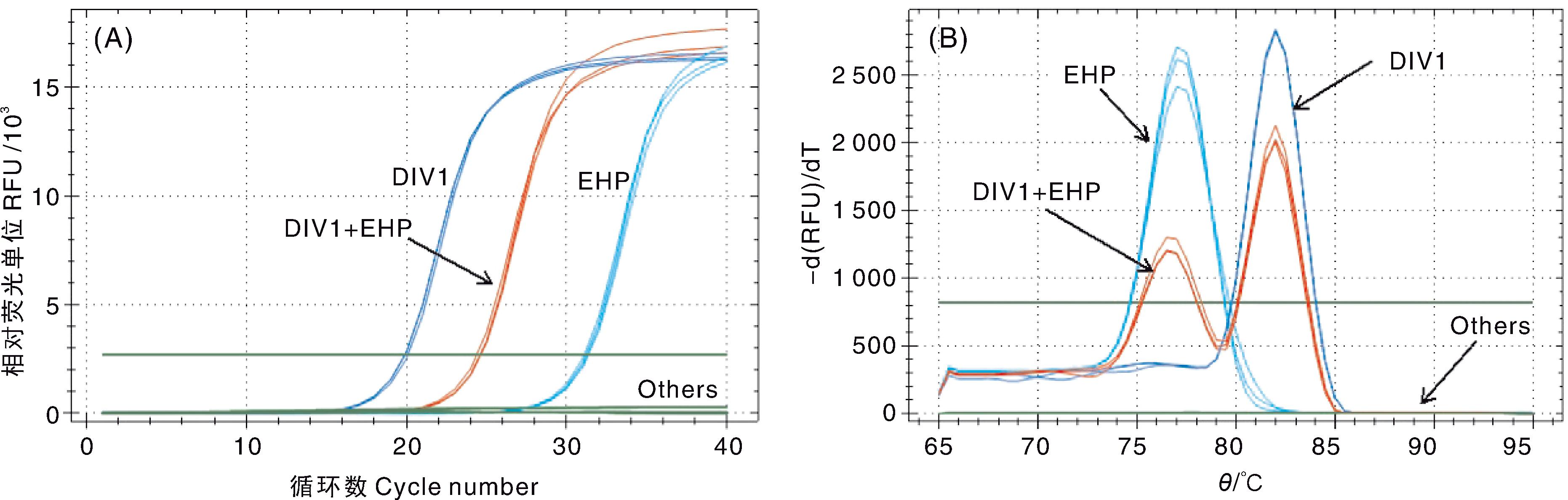
图7 双重SYBR Green I qPCR特异性试验结果 A,扩增曲线;B,熔解曲线。DIV1,DIV1阳性样本;EHP,EHP阳性样本;DIV1+EHP,混合感染样本;others,其他虾病阳性样本、健康凡纳滨对虾幼苗和阴性对照。
Fig.7 Analysis of the specificity of the double SYBR Green I qPCR A, Amplification curve; B, Melting curve. DIV1, DIV1 positive sample; EHP, EHP positive sample; DIV1+EHP, DIV1 and EHP co-infection sample; Others, Samples infected with other shrimp pathogens, healthy L. vannamei juveniles and negative control.
| pMD-MCP标准品拷贝数 Copies of the standard plasmid | 组内重复Intra-assay variability | 组间重复 Inter-assay variability | ||||||
|---|---|---|---|---|---|---|---|---|
| 第1天Day 1 | 第5天Day 5 | 第10天Day 10 | ||||||
| pMD-MCP/(copies·μL-1) | Cq | CV/% | Cq | CV/% | Cq | CV/% | Cq | CV/% |
| 7.5×107 | 13.43±0.12 | 0.92 | 13.77±0.06 | 0.46 | 13.35±0.03 | 0.19 | 13.52±0.21 | 1.52 |
| 7.5×106 | 16.80±0.02 | 0.10 | 17.04±0.12 | 0.68 | 17.00±0.06 | 0.35 | 16.94±0.13 | 0.76 |
| 7.5×105 | 20.46±0.06 | 0.31 | 20.52±0.02 | 0.09 | 20.40±0.02 | 0.10 | 20.46±0.06 | 0.30 |
| 7.5×104 | 23.90±0.05 | 0.21 | 24.35±0.08 | 0.33 | 24.04±0.07 | 0.30 | 24.09±0.21 | 0.86 |
| 7.5×103 | 27.21±0.08 | 0.31 | 27.55±0.06 | 0.21 | 27.54±0.08 | 0.27 | 27.43±0.18 | 0.64 |
| 7.5×102 | 30.65±0.15 | 0.50 | 31.12±0.02 | 0.06 | 30.94±0.02 | 0.05 | 30.90±0.22 | 0.71 |
| 7.5×101 | 33.75±0.04 | 0.12 | 34.12±0.02 | 0.05 | 33.62±0.08 | 0.25 | 33.83±0.23 | 0.68 |
表2 DIV1双重SYBR Green I qPCR检测的组内和组间重复性
Table 2 Intra-assay and inter-assay variability of the double SYBR Green I qPCR detection assay for DIV1
| pMD-MCP标准品拷贝数 Copies of the standard plasmid | 组内重复Intra-assay variability | 组间重复 Inter-assay variability | ||||||
|---|---|---|---|---|---|---|---|---|
| 第1天Day 1 | 第5天Day 5 | 第10天Day 10 | ||||||
| pMD-MCP/(copies·μL-1) | Cq | CV/% | Cq | CV/% | Cq | CV/% | Cq | CV/% |
| 7.5×107 | 13.43±0.12 | 0.92 | 13.77±0.06 | 0.46 | 13.35±0.03 | 0.19 | 13.52±0.21 | 1.52 |
| 7.5×106 | 16.80±0.02 | 0.10 | 17.04±0.12 | 0.68 | 17.00±0.06 | 0.35 | 16.94±0.13 | 0.76 |
| 7.5×105 | 20.46±0.06 | 0.31 | 20.52±0.02 | 0.09 | 20.40±0.02 | 0.10 | 20.46±0.06 | 0.30 |
| 7.5×104 | 23.90±0.05 | 0.21 | 24.35±0.08 | 0.33 | 24.04±0.07 | 0.30 | 24.09±0.21 | 0.86 |
| 7.5×103 | 27.21±0.08 | 0.31 | 27.55±0.06 | 0.21 | 27.54±0.08 | 0.27 | 27.43±0.18 | 0.64 |
| 7.5×102 | 30.65±0.15 | 0.50 | 31.12±0.02 | 0.06 | 30.94±0.02 | 0.05 | 30.90±0.22 | 0.71 |
| 7.5×101 | 33.75±0.04 | 0.12 | 34.12±0.02 | 0.05 | 33.62±0.08 | 0.25 | 33.83±0.23 | 0.68 |
| pMD-SWP标准品拷贝数 Copies of the standard plasmid | 组内重复Intra-assay variability | 组间重复 Inter-assay variability | ||||||
|---|---|---|---|---|---|---|---|---|
| 第1天Day 1 | 第5天Day 5 | 第10天Day 10 | ||||||
| pMD-SWP/(copies·μL-1) | Cq | CV/% | Cq | CV/% | Cq | CV/% | Cq | CV/% |
| 1.5×107 | 14.05±0.03 | 0.23 | 14.13±0.04 | 0.30 | 14.17±0.02 | 0.13 | 14.11±0.06 | 0.41 |
| 1.5×106 | 17.40±0.04 | 0.23 | 17.48±0.05 | 0.26 | 17.45±0.05 | 0.29 | 17.45±0.05 | 0.31 |
| 1.5×105 | 20.62±0.07 | 0.32 | 20.75±0.10 | 0.46 | 20.80±0.11 | 0.53 | 20.72±0.12 | 0.55 |
| 1.5×104 | 24.15±0.06 | 0.24 | 24.33±0.13 | 0.55 | 24.47±0.04 | 0.18 | 24.32±0.16 | 0.65 |
| 1.5×103 | 27.64±0.03 | 0.12 | 27.83±0.03 | 0.10 | 27.83±0.02 | 0.05 | 27.77±0.10 | 0.35 |
| 1.5×102 | 31.09±0.07 | 0.21 | 31.31±0.14 | 0.45 | 31.16±0.14 | 0.45 | 31.19±0.14 | 0.45 |
| 1.5×101 | 33.91±0.11 | 0.32 | 33.89±0.20 | 0.58 | 34.60±0.15 | 0.43 | 34.13±0.37 | 1.09 |
表3 EHP双重SYBR Green I qPCR检测的组内和组间重复性
Table 3 Intra-assay and inter-assay variability of the double SYBR Green I qPCR detection assay for EHP
| pMD-SWP标准品拷贝数 Copies of the standard plasmid | 组内重复Intra-assay variability | 组间重复 Inter-assay variability | ||||||
|---|---|---|---|---|---|---|---|---|
| 第1天Day 1 | 第5天Day 5 | 第10天Day 10 | ||||||
| pMD-SWP/(copies·μL-1) | Cq | CV/% | Cq | CV/% | Cq | CV/% | Cq | CV/% |
| 1.5×107 | 14.05±0.03 | 0.23 | 14.13±0.04 | 0.30 | 14.17±0.02 | 0.13 | 14.11±0.06 | 0.41 |
| 1.5×106 | 17.40±0.04 | 0.23 | 17.48±0.05 | 0.26 | 17.45±0.05 | 0.29 | 17.45±0.05 | 0.31 |
| 1.5×105 | 20.62±0.07 | 0.32 | 20.75±0.10 | 0.46 | 20.80±0.11 | 0.53 | 20.72±0.12 | 0.55 |
| 1.5×104 | 24.15±0.06 | 0.24 | 24.33±0.13 | 0.55 | 24.47±0.04 | 0.18 | 24.32±0.16 | 0.65 |
| 1.5×103 | 27.64±0.03 | 0.12 | 27.83±0.03 | 0.10 | 27.83±0.02 | 0.05 | 27.77±0.10 | 0.35 |
| 1.5×102 | 31.09±0.07 | 0.21 | 31.31±0.14 | 0.45 | 31.16±0.14 | 0.45 | 31.19±0.14 | 0.45 |
| 1.5×101 | 33.91±0.11 | 0.32 | 33.89±0.20 | 0.58 | 34.60±0.15 | 0.43 | 34.13±0.37 | 1.09 |
| 巢式PCR检测 Detection by nested-PCR | 双重SYBR Green I qPCR检测Detection by double SYBR Green I qPCR | |||
|---|---|---|---|---|
| 阳性Positive | 阴性Negative | 总计Total | ||
| DIV1 | 阳性Positive | 4 | 0 | 4 |
| 阴性Negative | 2 | 49 | 51 | |
| 总计Total | 6 | 49 | 55 | |
| EHP | 阳性Positive | 9 | 0 | 9 |
| 阴性Negative | 0 | 46 | 46 | |
| 总计Total | 9 | 46 | 55 | |
表4 临床样本使用巢式PCR和双重SYBR Green I qPCR检测的结果
Table 4 Detection results of clinical samples by nested-PCR and double SYBR Green I qPCR
| 巢式PCR检测 Detection by nested-PCR | 双重SYBR Green I qPCR检测Detection by double SYBR Green I qPCR | |||
|---|---|---|---|---|
| 阳性Positive | 阴性Negative | 总计Total | ||
| DIV1 | 阳性Positive | 4 | 0 | 4 |
| 阴性Negative | 2 | 49 | 51 | |
| 总计Total | 6 | 49 | 55 | |
| EHP | 阳性Positive | 9 | 0 | 9 |
| 阴性Negative | 0 | 46 | 46 | |
| 总计Total | 9 | 46 | 55 | |
| [1] | QIU L, CHEN M M, WAN X Y, et al. Characterization of a new member of Iridoviridae, Shrimp hemocyte iridescent virus (SHIV), found in white leg shrimp (Litopenaeus vannamei)[J]. Scientific Reports, 2017, 7: 11834. |
| [2] | XU L, WANG T, LI F, et al. Isolation and preliminary characterization of a new pathogenic iridovirus from redclaw crayfish Cherax quadricarinatus[J]. Diseases of Aquatic Organisms, 2016, 120(1): 17-26. |
| [3] | LI F, XU L M, YANG F. Genomic characterization of a novel iridovirus from redclaw crayfish Cherax quadricarinatus: evidence for a new genus within the family Iridoviridae[J]. Journal of General Virology, 2017, 98(10): 2589-2595. |
| [4] | QIU L, CHEN M M, WANG R Y, et al. Complete genome sequence of shrimp hemocyte iridescent virus (SHIV) isolated from white leg shrimp, Litopenaeus vannamei[J]. Archives of Virology, 2018, 163(3): 781-785. |
| [5] | CHEN X, QIU L, WANG H L, et al. Susceptibility of Exopalaemon carinicauda to the infection with shrimp hemocyte iridescent virus (SHIV 20141215), a strain of decapod iridescent virus 1 (DIV1)[J]. Viruses, 2019, 11(4): 387. |
| [6] | QIU L, CHEN X, ZHAO R H, et al. Description of a natural infection with decapod iridescent virus 1 in farmed giant freshwater prawn, Macrobrachium rosenbergii[J]. Viruses, 2019, 11(4): 354. |
| [7] | 潘长坤, 袁会芳, 王甜甜, 等. 红螯螯虾虹彩病毒在两种螃蟹内的研究[J]. 应用海洋学学报, 2017, 36(1): 82-86. |
| PAN C K, YUAN H F, WANG T T, et al. Study of Cherax quadricarinatus iridescent virus in two crabs[J]. Journal of Applied Oceanography, 2017, 36(1): 82-86. (in Chinese with English abstract) | |
| [8] | SRISALA J, SANGUANRUT P, THAIUE D, et al. Infectious myonecrosis virus (IMNV) and decapod iridescent virus 1 (DIV1) detected in captured, wild Penaeus monodon[J]. Aquaculture, 2021, 545: 737262. |
| [9] | CHAYABURAKUL K, NASH G, PRATANPIPAT P, et al. Multiple pathogens found in growth-retarded black tiger shrimp Penaeus monodon cultivated in Thailand[J]. Diseases of Aquatic Organisms, 2004, 60: 89-96. |
| [10] | TOURTIP S, WONGTRIPOP S, STENTIFORD G D, et al. Enterocytozoon hepatopenaei sp. nov. (Microsporida: Enterocytozoonidae), a parasite of the black tiger shrimp Penaeus monodon(Decapoda: Penaeidae): fine structure and phylogenetic relationships[J]. Journal of Invertebrate Pathology, 2009, 102(1): 21-29. |
| [11] | ARANGUREN L F, HAN J E, TANG K F J. Enterocytozoon hepatopenaei(EHP) is a risk factor for acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN) in the Pacific white shrimp Penaeus vannamei[J]. Aquaculture, 2017, 471: 37-42. |
| [12] | CHAIJARASPHONG T, MUNKONGWONGSIRI N, STENTIFORD G D, et al. The shrimp microsporidian Enterocytozoon hepatopenaei(EHP): biology, pathology, diagnostics and control[J]. Journal of Invertebrate Pathology, 2021, 186: 107458. |
| [13] | TANG K F J, HAN J E, ARANGUREN L F, et al. Dense populations of the microsporidian Enterocytozoon hepatopenaei(EHP) in feces of Penaeus vannamei exhibiting white feces syndrome and pathways of their transmission to healthy shrimp[J]. Journal of Invertebrate Pathology, 2016, 140: 1-7. |
| [14] | SALACHAN P V, JAROENLAK P, THITAMADEE S, et al. Laboratory cohabitation challenge model for shrimp hepatopancreatic microsporidiosis (HPM) caused by Enterocytozoon hepatopenaei(EHP)[J]. BMC Veterinary Research, 2017, 13(1): 9. |
| [15] | CARO L F A, MAI H N, SCHOFIELD P, et al. A laboratory challenge model for evaluating Enyterocytozoon hepatopenaei susceptibility in selected lines of Pacific whiteleg shrimp Penaeus vannamei[J]. Journal of Invertebrate Pathology, 2023, 196: 107853. |
| [16] | 刘珍, 张庆利, 万晓媛, 等. 虾肝肠胞虫(Enterocytozoon hepatopenaei)实时荧光定量PCR检测方法的建立及对虾样品的检测[J]. 渔业科学进展, 2016, 37(2): 119-126. |
| LIU Z, ZHANG Q L, WAN X Y, et al. Development of real-time PCR assay for detecting microsporidian Enterocytozoon hepatopenaei and the application in shrimp samples with different growth rates[J]. Progress in Fishery Sciences, 2016, 37(2): 119-126. (in Chinese with English abstract) | |
| [17] | RAJENDRAN K V, SHIVAM S, EZHIL PRAVEENA P, et al. Emergence of Enterocytozoon hepatopenaei(EHP) in farmed Penaeus (Litopenaeus) vannamei in India[J]. Aquaculture, 2016, 454: 272-280. |
| [18] | BIJU N, SATHIYARAJ G, RAJ M, et al. High prevalence of Enterocytozoon hepatopenaei in shrimps Penaeus monodon and Litopenaeus vannamei sampled from slow growth ponds in India[J]. Diseases of Aquatic Organisms, 2016, 120(3): 225-230. |
| [19] | WAN SAJIRI W M H, KUA B C, BORKHANUDDIN M H. Detection of Enterocytozoon hepatopenaei(EHP) (microsporidia) in several species of potential macrofauna-carriers from shrimp (Penaeus vannamei) ponds in Malaysia[J]. Journal of Invertebrate Pathology, 2023,198: 107910. |
| [20] | TANG K F J, PANTOJA C R, REDMAN R M, et al. Development of in situ hybridization and PCR assays for the detection of Enterocytozoon hepatopenaei(EHP), a microsporidian parasite infecting penaeid shrimp[J]. Journal of Invertebrate Pathology, 2015, 130: 37-41. |
| [21] | 农业农村部渔业渔政管理局, 全国水产技术推广总站. 2020我国重要水生动物疫病状况分析[M]. 北京: 中国农业出版社, 2020. |
| [22] | JAROENLAK P, SANGUANRUT P, WILLIAMS B A P, et al. A nested PCR assay to avoid false positive detection of the microsporidian Enterocytozoon hepatopenaei(EHP) in environmental samples in shrimp farms[J]. PLoS One, 2016, 11(11): e0166320. |
| [23] | CHEN Z W, HUANG J, ZHANG F, et al. Detection of shrimp hemocyte iridescent virus by recombinase polymerase amplification assay[J]. Molecular and Cellular Probes, 2020, 49: 101475. |
| [24] | LI G, CONG F, CAI W Y, et al. Development of a recombinase polymerase amplification (RPA) fluorescence assay for the detection of Enterocytozoon hepatopenaei(EHP)[J]. Aquaculture Reports, 2021, 19: 100584. |
| [25] | TONG G X, YIN W L, WU X Q, et al. Rapid detection of decapod iridescent virus 1 (DIV1) by recombinase polymerase amplification[J]. Journal of Virological Methods, 2022, 300: 114362. |
| [26] | T S K, A N K, J J S R, et al. Visual loop-mediated isothermal amplification (LAMP) for the rapid diagnosis of Enterocytozoon hepatopenaei(EHP) infection[J]. Parasitology Research, 2018, 117(5): 1485-1493. |
| [27] | GONG H Y, LI Q Y, ZHANG H, et al. Development and comparison of qPCR and qLAMP for rapid detection of the decapod iridescent virus 1 (DIV1)[J]. Journal of Invertebrate Pathology, 2021, 182: 107567. |
| [28] | LIU Y M, QIU L, SHENG A Z, et al. Quantitative detection method of Enterocytozoon hepatopenaei using TaqMan probe real-time PCR[J]. Journal of Invertebrate Pathology, 2018, 151: 191-196. |
| [29] | QIU L, CHEN M M, WAN X Y, et al. Detection and quantification of shrimp hemocyte iridescent virus by TaqMan probe based real-time PCR[J]. Journal of Invertebrate Pathology, 2018, 154: 95-101. |
| [30] | XU T, TAN R X, ZHU Y T, et al. Establishment of a SYBR Green I-based real-time PCR for the detection of decapod iridescent virus 1[J]. Journal of Invertebrate Pathology, 2023, 201: 107998. |
| [31] | QIU L, CHEN X, GUO X M, et al. A TaqMan probe based real-time PCR for the detection of Decapod iridescent virus 1[J]. Journal of Invertebrate Pathology, 2020, 173: 107367. |
| [32] | 侯月娥, 曾俊霞, 蓝间媛, 等. EHP、SHIV双重TaqMan实时荧光定量PCR检测方法的构建及应用[J]. 中国动物传染病学报, 2022, 30(1): 112-119. |
| HOU Y E, ZENG J X, LAN J Y, et al. Development of a duplex TaqMan real-time PCR assay for detection of Enterocytozoon hepatopenaei and shrimp hemocyte iridovirus[J]. Chinese Journal of Veterinary Parasitology, 2022, 30(1): 112-119. (in Chinese with English abstract) | |
| [33] | 汪浩, 汪玮, 施慧, 等. 基于孢子壁蛋白基因靶点的虾肝肠胞虫实时荧光定量PCR体系的构建及应用[J]. 浙江海洋大学学报(自然科学版), 2020(6): 477-483. |
| WANG H, WANG W, SHI H, et al. Establishment and application of a real-time PCR assay targeted on the spore wall protein gene for detecting microsporidian Enterocytozoon hepatopenaei[J]. Journal of Zhejiang Ocean University(Natural Science), 2020(6): 477-483. (in Chinese with English abstract) |
| [1] | 周聃, 刘梅, 张政, 邹松保, 倪蒙, 原居林. 虾蟹混养池塘的温室气体排放及其影响因子[J]. 浙江农业学报, 2025, 37(9): 1872-1880. |
| [2] | 任晋东, 陈红林, 牛宝龙, 许晓军, 楼宝. 基于转录组分析挖掘罗氏沼虾新内参基因[J]. 浙江农业学报, 2025, 37(7): 1424-1429. |
| [3] | 黄贤克, 黄晓林, 张翔, 李敏, 蔡逸龙, 陈然. 牡蛎壳对凡纳对虾生长性能和水质的作用效果及其表面菌群特征[J]. 浙江农业学报, 2025, 37(7): 1441-1450. |
| [4] | 翁歆之, 刁奕昕, 贺洁, 刘莉, 沈海钰, 郭琦, 沈卫锋, 韩明明, 楼宝, 吕孙建. 弧菌噬菌体鸡尾酒制剂对凡纳滨对虾肠道微生物区系的影响[J]. 浙江农业学报, 2025, 37(5): 1045-1056. |
| [5] | 唐金玉, 黄福勇, 戴杨鑫, 楼宝, 郭水荣. 稻-红螯螯虾共作模式下浮游生物的群落变化特征及其与虾生长之间的关系[J]. 浙江农业学报, 2024, 36(7): 1537-1547. |
| [6] | 彭佳诚, 吴越, 徐洁皓, 夏美文, 齐天鹏, 徐海圣. 日本沼虾桩蛋白基因的克隆与镉胁迫对其表达的影响[J]. 浙江农业学报, 2024, 36(2): 247-253. |
| [7] | 唐金玉, 覃宝利, 叶建勇, 戴杨鑫. 放养模式对日本沼虾生长性状与肌肉营养成分的影响[J]. 浙江农业学报, 2024, 36(2): 254-263. |
| [8] | 刘芳芳, 陈红林, 刘峰, 许晓军, 黄福勇, 楼宝, 钱豪杰. 雌雄红螯螯虾染色体核型比较分析[J]. 浙江农业学报, 2023, 35(9): 2079-2089. |
| [9] | 梁妃爽, 梁华芳, 黄佳宇, 王潘妹, 温崇庆. RNA干扰PhCatC1/2基因对波纹龙虾相关免疫基因表达的影响[J]. 浙江农业学报, 2023, 35(5): 1037-1047. |
| [10] | 郝柳柳, 代梨梨, 彭亮, 陈思媛, 陶玲, 李谷, 张辉. 稻虾种养系统水稻根际土壤活性有机碳、微生物群落结构及其相互关系[J]. 浙江农业学报, 2023, 35(12): 2901-2913. |
| [11] | 陈乐然, 郑建波, 贾永义, 迟美丽, 李飞, 程顺, 刘士力, 刘一诺, 蒋文枰, 顾志敏. 红螯螯虾CHH2基因的表达特征及其在卵巢发育中的功能[J]. 浙江农业学报, 2023, 35(1): 33-40. |
| [12] | 董靖, 张露珊, 张国栋, 刘永涛, 刘绍春, 杨移斌, 艾晓辉. 一株克氏原螯虾雷氏普罗威登斯菌的分离鉴定与联合药敏试验[J]. 浙江农业学报, 2022, 34(1): 24-32. |
| [13] | 刘士力, 卞玉玲, 贾永义, 迟美丽, 李飞, 郑建波, 程顺, 顾志敏. 基于线粒体CO Ⅰ 基因序列的红螯螯虾养殖群体遗传结构分析[J]. 浙江农业学报, 2021, 33(8): 1385-1392. |
| [14] | 沈卫锋, 郭琦, 刘莉, 牛宝龙, 翁宏飚, 楼宝. 虾肝肠胞虫(Enterocytozoon hepatopenaei)SWP2基因的克隆、表达及其在虾类病害检测中的应用[J]. 浙江农业学报, 2021, 33(6): 993-1000. |
| [15] | 厉宝仙, 王保君, 怀燕, 沈亚强, 张红梅, 程旺大. 水稻-红鳌螯虾共作对稻田土壤养分、碳库与稻米品质的影响[J]. 浙江农业学报, 2021, 33(4): 688-696. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||